Cartilage Regeneration in Osteoarthritic Patients by a Composite of Allogeneic Umbilical Cord Blood‐Derived Mesenchymal Stem Cells and Hyaluronate Hydrogel
Results from a Clinical Trial for Safety and Proof‐of‐Concept with 7 Years of Extended Follow‐Up
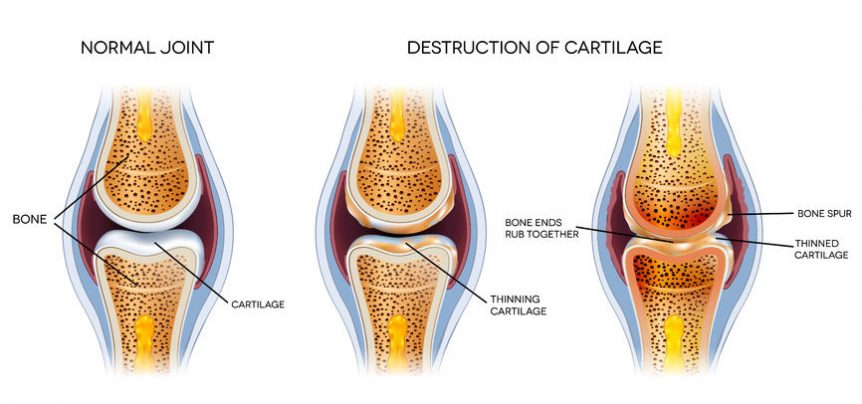
Few methods are available to regenerate articular cartilage defects in patients with osteoarthritis. We aimed to assess the safety and efficacy of articular cartilage regeneration by a novel medicinal product composed of allogeneic human umbilical cord blood‐derived mesenchymal stem cells (hUCB‐MSCs). Patients with Kellgren‐Lawrence grade 3 osteoarthritis and International Cartilage Repair Society (ICRS) grade 4 cartilage defects were enrolled in this clinical trial. The stem cell‐based medicinal product (a composite of culture‐expanded allogeneic hUCB‐MSCs and hyaluronic acid hydrogel [Cartistem]) was applied to the lesion site. Safety was assessed by the World Health Organization common toxicity criteria. The primary efficacy outcome was ICRS cartilage repair assessed by arthroscopy at 12 weeks.