WHAT IS CHELATION THERAPY?
The term chelation (pronounced key-lay-shun) is derived from “chelos”, the Greek word for claw. Chelation therapy is an established treatment, used in conventional medicine for removing heavy metals and in the treatment of cardiovascular disease. In addition, chelation therapy has been used in the treatment of autoimmune, neurodegenerative and other diseases.
The purpose of chelation therapy is to bind minerals and/or toxic metals in the body with a chelating agent in order to enhance elimination and, therefore detoxify the body. Of primary concern are calcium deposits in arteries, causing atherosclerosis, a serious risk for heart attack and stroke, and heavy metals such as lead, mercury, arsenic, and others.
A number of different chelating agents are used in chelation therapy. Your doctor will choose the appropriate one depending on the mineral or heavy metal to be chelated. For example, 2,3-Dimercapto-1-1propanesulfonic acid (DMPS) is commonly used in reducing mercury levels and Ethylenediaminetetraacetic acid (EDTA) is used in treating atherosclerosis and coronary artery disease. Your doctor may also recommend natural agents, such as dietary fiber, cilantro, chlorella, etc., to be taken during your treatment for increased chelation and detoxification support.
A number of therapeutic agents such as N-acetyl-cysteine (NAC), Alpha Lipoic acid (ALA), Selenium, B vitamins, and others, are used in combination with chelation therapy, to provide antioxidant support and to optimize detoxification. In addition, replenishing minerals is imperative while receiving chelation therapy as the chelating agents can bind essential minerals. Our Chelation Protocol includes this additional support using oral supplements and intravenous nutrients.
WHAT IS CHE-ZONE THERAPY?
Che-Zone Therapy, a procedure created by Dr. Frank Shallenberger, M.D., combines intravenous chelation therapy with intravenous ozone therapy. The addition of ozone therapy maximizes the detoxification effect and further supports liver function. This supports increased elimination of heavy metals, toxins and other substances. Intravenous ozone is typically given with glutathione and N-acetylcysteine prior to the chelation IV.
HEAVY METALS
Heavy metals such as lead, mercury, and arsenic are toxic to the body. High levels of these toxic metals in the body may be associated with a wide range of debilitating symptoms. Lead burden can lead to higher rates of Parkinsonism and cognitive decline in adults, and learning, and behavioral difficulties in children. Mercury exposure is associated with mood disturbances, cardiovascular conditions such as high blood pressure, infertility and immune dysfunction. Arsenic is a known carcinogen and can increase the risk for diabetes.
Anatara Medicine uses questionnaires, physical examinations and laboratory testing to evaluate patients for heavy metal burden. Laboratory testing include assessment of heavy metals in blood and urine, using Doctor’s Data Laboratory.
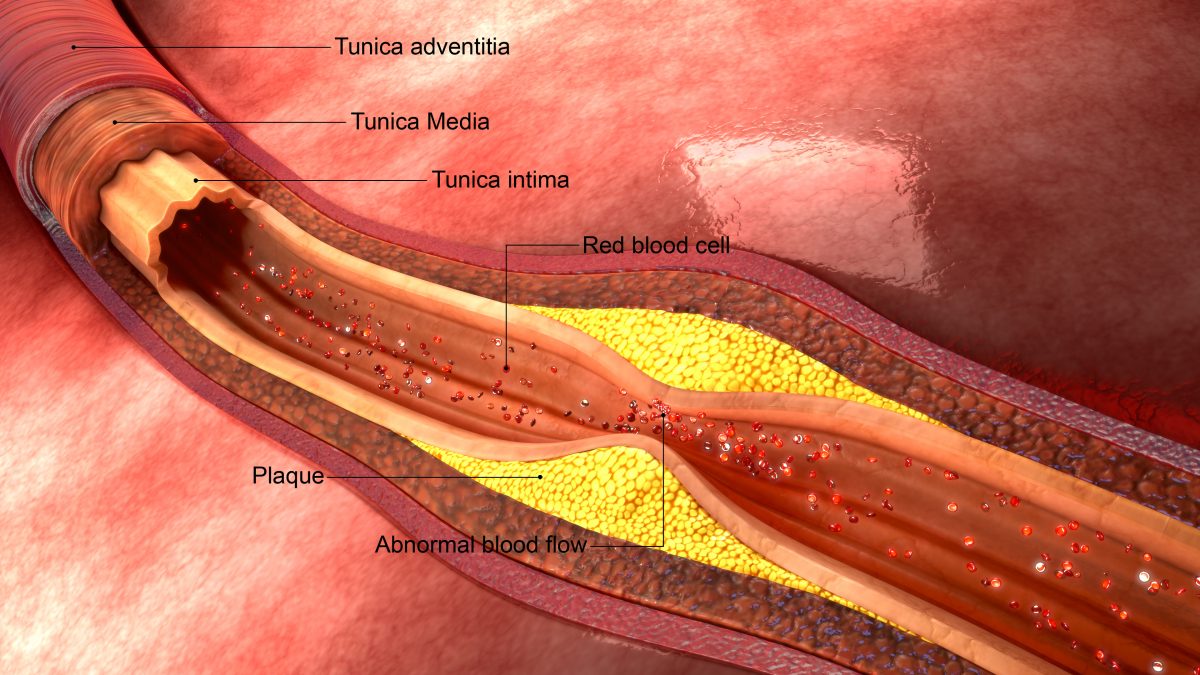
CARDIOVASCULAR DISEASE
Chelation therapy has also been used in the treatment of cardiovascular. TACT (Trial to Assess Chelation Therapy) is the first large scale study to determine the efficacy and safety of chelation therapy, with or without high-dose vitamins, for those with cardiovascular disease including prior heart attacks, coronary artery disease, diabetes, etc. The preliminary results of this 10 year, $31 million study show that EDTA chelation therapy produced modest, but statistically significant reductions in cardiovascular events. Patients with diabetes were more positively impacted showing a 40% reduction in risk of death from heart disease, 52% reduction in recurrent heart attacks and 43% reduction in death from any cause. Gervasio Lamas, M.D., the study’s principal investigator, remarked “These are striking results that, if supported by future research, could point the way towards new treatments to prevent complications of diabetes”.
OTHER CONDITIONS
Research studies investigating the implications of chelation therapy on autism, neurodegenerative diseases such as Alzheimer’s disease, multiple sclerosis, and Parkinson’s disease, macular degeneration, rheumatoid arthritis and others have been published. See below for links to research and literature on chelation therapy.
HOW IS CHELATION ADMINISTERED?
Chelation therapy can be administered intravenously, rectally and/or orally. The route of administration is chosen depending on the objective of the treatment, the type of heavy metal to be chelated and patient tolerability. For example, EDTA is commonly administered intravenously. Oral administration can be performed at home while intravenous administration is performed in our office.
IS IT SAFE? ARE THERE SIDE EFFECTS?
When properly performed chelation therapy is safe, and well tolerated. The treatment protocols for chelation therapy are used by chelation therapy practitioners worldwide and have been rigorously tested.
Research has shown that the Lethal Dose 50 (LD50: the dose that will kill 50% of all test animals) of EDTA is 1900mg per kilogram of body weight, while the LD50 of aspirin is 420mg per kilogram of body weight. This means that the LD50 of EDTA is 88% higher than that of aspirin.
Chelation therapy is typically well tolerated with minimal side effects. The most common side effect is mild bleeding, bruising and burning sensation at the site of injection. Our center takes extra precautions to reduce this and ensure the patient is comfortable throughout the treatment. Rarely, short-term adverse side effects may occur. These include fever, headache, malaise, digestive upset including diarrhea, bloating, nausea and vomiting. If these occur, notify your doctor and they will recommend additional treatments. These side effects resolve when treatment is stopped.
Chelation therapy is not indicated for everyone. People with kidney disease, receiving renal dialysis and who are pregnant or breast feeding should absolutely not get chelation therapy. Your doctor will perform a thorough assessment and formulate a treatment plan for you.
HOW DO I KNOW IF I HAVE ELEVATED LEVELS OF HEAVY METALS? HOW DO I GET TESTED?
Anatara Medicine uses the most accurate and highest quality laboratories to assess heavy metal burden. Assessment of your heavy metals will be done with symptom questionnaires, physical examinations and laboratory testing using blood and urine.
HOW DO I KNOW IF I HAVE CLOGGED ARTERIES?
There are many symptoms of clogged arteries (atherosclerosis), such as high blood pressure, chest pain, difficulty breathing, fatigue, and many more. Your doctor may have already diagnosed you with atherosclerosis, coronary artery disease, or other cardiovascular disease. Common tests include calcium score, angiography, and Chorus CAD. If needed, Anatara Medicine will perform comprehensive testing to evaluate your cardiovascular health including specialty blood work and imaging.
WHAT SHOULD I EXPECT ON MY FIRST VISIT?
The doctors of Anatara Medicine will complete a thorough history of your health concerns, assess your symptoms using validated questionnaires, perform physical exams and order appropriate comprehensive laboratory testing. Chelation therapy may be given intravenously (IV) or orally, depending on the heavy metal, treatment objective, and patient tolerance. Intravenous chelation is performed in our office and involves receiving multiple IV treatments of the selected chelating agent and nutrients. Unfortunately, it is not possible to know the exact number of treatments you will need. We use symptom questionnaires and repeat testing to monitor progress and assess treatment success. If you are going to receive heavy metal chelation, your first treatment is also your first heavy metal test. On the day of your heavy metal chelation treatment you will provide a before chelation and after chelation urine specimen that will be used to assess your level of heavy metal burden. This test is done through Doctor’s Data, one of the most respected and most common laboratories to assess heavy metals in urine.
HOW LONG DOES IV CHELATION TAKE?
IV chelation for cardiovascular disease typically takes 2-3 hours. IV chelation for heavy metals typically takes 1 hour. Additional IV therapies may be recommended and can be completed on the same day as your chelation treatment. In most cases multiple treatments over a number of months is necessary to improve cardiovascular health and to reduce heavy metal burden.
IS CHELATION THERAPY AN ALTERNATIVE TO CORONARY ARTERY BYPASS SURGERY (CABG) AND OTHER CONVENTIONAL TREATMENTS?
There is early data that suggests that cardiovascular patients treated with EDTA chelation therapy have a lower rate of subsequent cardiac events, including myocardial infarction and death, than those treated with cardiac medications, Percutaneous Transluminal Coronary Angioplasty (PTCA), or Coronary Artery Bypass Graft (CABG). Data also suggests that chelation therapy might be effective in preventing blood clots and cardiac events from stents by reducing hypercoagulability.
IS THERE ANY RESEARCH ON CHELATION THERAPY?
TACT (Trial to Assess Chelation Therapy) is the first large scale study to determine the efficacy and safety of chelation therapy, with or without high-dose vitamins, for those with cardiovascular disease including prior heart attacks, coronary artery disease, diabetes, etc. The preliminary results of this 10 year, $31 million study show that EDTA chelation therapy produced modest, but statistically significant reductions in cardiovascular events. Patients with diabetes were more positively impacted showing a 40% reduction in risk of death from heart disease, 52% reduction in recurrent heart attacks and 43% reduction in death from any cause. Gervasio Lamas, M.D., the study’s principal investigator, remarked “These are striking results that, if supported by future research, could point the way towards new treatments to prevent complications of diabetes”. **See below for links to research and literature on chelation therapy.
IS CHELATION THERAPY FDA APPROVED?
The FDA has approved 11 chelating agents, including EDTA and DMSA, to treat heavy metal toxicities. Currently, chelation therapy is not approved by the FDA to treat other diseases, despite scientific research that supports its use in a variety of diseases. (Wax PM. Current Use of Chelation in American Health Care. Journal of Medical Toxicology. 2013;9(4):303-307. doi:10.1007/s13181-013-0347-2).
HOW COMMON IS CHELATION THERAPY?
Practitioners all over the world use chelation therapy in for variety of conditions. A 2008 National Health Statistics Report estimates that in 2002, 66,000 adults received chelation therapy. This same report estimated that 111,000 adults and 72,00 children received some sort of chelation therapy 2007. (Wax PM. Current Use of Chelation in American Health Care. Journal of Medical Toxicology. 2013;9(4):303-307. doi:10.1007/s13181-013-0347-2)
CONDITIONS TREATED WITH CHELATION THERAPY
- Heavy Metals and Other Toxins
- Lead
- Mercury
- Arsenic
- Aluminum
- Cadmium
- Antimony
- Tin
- Thallium
- Iron
- Gadolinium
- Cardiovascular Disease
- High blood pressure (Hypertension)
- Clogged/harden arteries (Atherosclerosis)
- Coronary artery disease
- Diabetes
- Autoimmune & Neurodegenerative Diseases
- Alzheimer’s disease
- Parkinson’s disease
- Multiple sclerosis
- Rheumatoid arthritis
- Autism
- Eye Disease
- Macular Degeneration – Age Related (AMD)
SOURCES/LITERATURE/RESEARCH
HEAVY METALS
Flushing Out Lead, Metals With Chelation Therapy – http://www.npr.org/2011/01/03/132474747/flushing-out-lead-metals-with-chelation-therapy
Metal pollutants and cardiovascular disease – mechanisms and consequences of exposure – http://www.ncbi.nlm.nih.gov/pubmed/25458643
Heavy Metal Poisoning – National Organization for Rare Disorders – http://rarediseases.org/rare-diseases/heavy-metal-poisoning/
Heavy Metals in the work place – OSHA – https://www.osha.gov/SLTC/metalsheavy/
Hazards of heavy metal contamination – http://www.ncbi.nlm.nih.gov/pubmed/14757716
Heavy Metal Toxins a Danger in Homes – http://www.washingtonpost.com/wp-dyn/content/article/2007/01/20/AR2007012000443.html
2,3-Dimercaptosuccinic acid treatment of heavy metal poisoning in humans – http://www.ncbi.nlm.nih.gov/pubmed/2851085/
Use of oral dimercaptosuccinic acid (succimer) in adult patients with inorganic lead poisoning. – http://www.ncbi.nlm.nih.gov/pubmed/19700440
Metals and women’s health – http://www.ncbi.nlm.nih.gov/pubmed/12051792
Dietary cadmium exposure and risk of breast, endometrial, and ovarian cancer in the Women’s Health Initiative – http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4050510/pdf/ehp.1307054.pdf
Adverse effects of low level heavy metal exposure on male reproductive function – http://www.ncbi.nlm.nih.gov/pubmed/20377313
CARDIOVASCULAR
Questions and Answers: The NIH Trial of EDTA Chelation Therapy for Coronary Heart Disease – http://www.nhlbi.nih.gov/news/press-releases/supplement/questions-and-answers-the-nih-trial-of-edta-chelation-therapy-for-coronary-heart-disease
Chelation for coronary heart disease – https://nccih.nih.gov/health/chelation
Chelation therapy and cardiovascular disease: connecting scientific silos to benefit cardiac patients – http://www.ncbi.nlm.nih.gov/pubmed/25106084
Chelation Therapy Reduces Cardiovascular Events for Older Patients with Diabetes – https://nccih.nih.gov/news/2013/111913
EDTA chelation therapy modestly reduces cardiovascular events – https://www.nih.gov/news-events/news-releases/edta-chelation-therapy-modestly-reduces-cardiovascular-events
Chelation therapy for atherosclerotic cardiovascular disease – http://www.ncbi.nlm.nih.gov/pubmed/12519577
NIH statement on the vitamin component of the Trial to Assess Chelation Therapy – http://www.nhlbi.nih.gov/news/press-releases/2013/nih-statement-on-the-vitamin-component-of-the-trial-to-assess-chelation-therapy
TACT: Surprising, Puzzling Benefit from Chelation Therapy After MI – http://www.medscape.com/viewarticle/773885#vp_2
Quality-of-life outcomes with a disodium EDTA chelation regimen for coronary disease: results from the trial to assess chelation therapy randomized trial – https://www.ncbi.nlm.nih.gov/pubmed/24987051?dopt=Abstract
The effect of an EDTA-based chelation regimen on patients with diabetes mellitus and prior myocardial infarction in the Trial to Assess Chelation Therapy (TACT) – https://www.ncbi.nlm.nih.gov/pubmed/24254885?dopt=Abstract
Effect of disodium EDTA chelation regimen on cardiovascular events in patients with previous myocardial infarction: the TACT randomized trial – https://www.ncbi.nlm.nih.gov/pubmed/23532240?dopt=Abstract
Oral high-dose multivitamins and minerals after myocardial infarction: a randomized trial – https://www.ncbi.nlm.nih.gov/pubmed/24490264?dopt=Abstract
Should EDTA chelation therapy be used instead of long-term clopidogrel plus aspirin to treat patients at risk from drug-eluting stents – http://www.ncbi.nlm.nih.gov/pubmed/17604460
OTHERS
Chelation therapy for neurodegenerative diseases – http://www.ncbi.nlm.nih.gov/pubmed/19177468
Current Use of Chelation in American Health Care – http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3846961/
Iron chelation and multiple sclerosis – http://www.ncbi.nlm.nih.gov/pubmed/24397846
Iron chelation in the treatment of neurodegenerative disease – http://www.ncbi.nlm.nih.gov/pubmed/27033472
Elevated urinary excretion of aluminum and iron in multiple sclerosis – http://www.ncbi.nlm.nih.gov/pubmed/17086897